What is hard water

What is hard water ?
Hard water is water that contains a high concentration of dissolved minerals, primarily calcium and magnesium. These minerals are naturally picked up as water moves through soil and rock, especially limestone and chalk deposits, before reaching wells, aquifers, or municipal water systems.
Hard water is very common around the world and affects millions of households.
How Hard Water Forms
When rainwater falls, it is naturally soft. As it travels through the ground, it dissolves minerals from surrounding rock and soil. Areas with limestone or mineral-rich geology tend to have harder water.
The longer water stays underground, the more minerals it absorbs, which is why groundwater sources usually produce harder water than surface water from lakes or rivers.
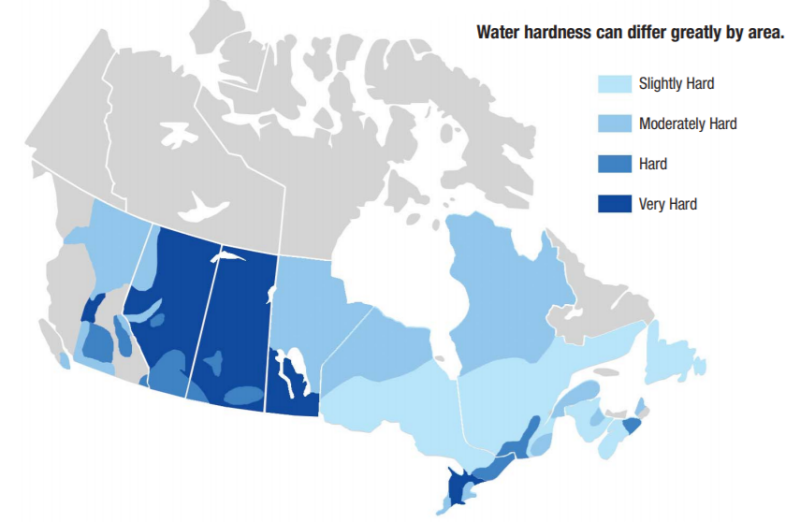
How Water Hardness Is Measured
Water hardness is measured in:
-
Milligrams per litre (mg/L)
-
Parts per million (ppm)
-
Grains per gallon (gpg)
General classifications:
-
Soft: 0–60 mg/L (0–3.5 gpg)
-
Moderately hard: 61–120 mg/L (3.6–7 gpg)
-
Hard: 121–180 mg/L (7–10.5 gpg)
-
Very hard: Over 180 mg/L (10.5+ gpg)
Get in touch with us today to get your Free water test
Common Signs of Hard Water
Homes with hard water often experience:
White, chalky scale buildup on faucets and showerheads
-
Soap scum on tubs, sinks, and tiles
-
Soap and shampoo that don’t lather well
-
Spots on dishes and glassware
-
Dry skin and dull or stiff-feeling hair
-
Reduced water flow due to scale in pipes
Effects of Hard Water on the Home
Plumbing and Appliances
Hard water minerals form scale inside:
-
Water heaters
-
Boilers
-
Dishwashers
-
Washing machines
-
Humidifiers
-
Pipes and valves
This buildup reduces efficiency, increases energy use, and shortens the lifespan of equipment.
Cleaning and Laundry
-
More soap and detergent is required
-
Clothes may feel stiff or fade faster
-
Surfaces require more frequent cleaning
Energy Costs
Scale acts as an insulator inside water heaters, forcing them to work harder to heat water. Over time, this can lead to higher utility bills.
Health Considerations
Hard water is not harmful to drink. Calcium and magnesium are essential minerals and may even contribute small nutritional benefits. However, very hard water can:
-
Affect taste
-
Leave residue in beverages like tea and coffee
-
Cause skin irritation for some people
Hard Water vs. Soft Water
-
Hard water: High calcium and magnesium
-
Soft water: Minerals have been removed or reduced, usually through a water softener
Soft water improves soap efficiency, protects plumbing, and reduces scale buildup.
How Hard Water Is Treated
The most common solution is a water softener, which uses an ion-exchange process to remove calcium and magnesium and replace them with sodium or potassium ions.
Other options include:
-
Salt-free conditioners (reduce scale but don’t remove minerals)
-
Point-of-use filters (for drinking water taste)
-
Descaling systems (help reduce buildup but don’t soften water)
Why Hard Water Matters
While hard water is safe, it can:
-
Increase household maintenance costs
-
Reduce appliance life
-
Increase energy consumption
-
Make everyday cleaning more difficult
SEE WATER HARDNESS IN YOUR CITY
1. Paris, Ontario
-
Hardness: ~37–43 gpg
-
Softener Regeneration Burden: Very High
2. Fergus, Ontario
-
Hardness: ~38 gpg
-
Softener Regeneration Burden: Very High
3. Kitchener, Ontario
-
Hardness: ~17–38 gpg
-
Softener Regeneration Burden: High to Very High
4. Waterloo, Ontario
-
Hardness: ~17–38 gpg
-
Softener Regeneration Burden: High to Very High
5. Cambridge, Ontario
-
Hardness: ~21–33 gpg
-
Softener Regeneration Burden: High
6. Guelph, Ontario
-
Hardness: ~21–33 gpg
-
Softener Regeneration Burden: High
7. Elora, Ontario
-
Hardness: ~30 gpg
-
Softener Regeneration Burden: High
8. St. Jacobs, Ontario
-
Hardness: ~19–28+ gpg
-
Softener Regeneration Burden: Moderate to High
9. Brantford, Ontario
-
Hardness: ~18–22 gpg
-
Softener Regeneration Burden: Moderate to High
10. Puslinch, Ontario
-
Hardness: ~20–30+ gpg
-
Softener Regeneration Burden: Moderate to High
11. Woodstock, Ontario
-
Hardness: ~20+ gpg
-
Softener Regeneration Burden: Moderate
12. Georgetown, Ontario
-
Hardness: ~23.6 gpg
-
Softener Regeneration Burden: Moderate
Here is the solution for your hard water and here is why it works
A water softener works through a scientific process called ion exchange. Inside the system is a tank filled with millions of tiny, negatively charged resin beads. As hard water (which is high in calcium and magnesium) flows through the tank, these minerals—which carry a positive charge—are attracted to the beads like magnets and stick to them. To maintain a balance, the beads release a small amount of sodium or potassium into the water. This effectively "swaps" the minerals that cause scale buildup for harmless salt ions, resulting in soft water that is gentler on your plumbing, skin, and appliances.





Add comment
Comments